The Tomato Fruit Cuticular Cell Wall Proteome
The plant cuticle is a highly specialized hydrophobic cell wall that covers the aerial organs of land plants and provides protection against desiccation, pathogens, UV radiation and herbivory. It is continuous with the outer-periclinal polysaccharide cell wall of the epidermis and consists of organic soluble waxes embedded in, and layered on, a non-soluble polyester matrix of ω-substituted fatty acids. The waxes include both aliphatic compounds, derived from very long chain fatty acids, and secondary metabolites, such as triterpenoids and flavonoids.
Little is yet known about many key mechanisms of cuticle biogenesis, and experimental strategies to identify new proteins that associate with cutin and waxes could provide a valuable means to identify new candidates.
Although Arabidopsis research has greatly accelerated the discovery of new cuticle-related genes, its cuticle poses some experimental limitations since it is relatively thin, fragile and difficult to isolate in substantial quantities. Conversely, tomato fruit cuticles are astomatous and large amounts of intact cuticular material can be isolated for chemical and biomechanical analyses. For example, the fruit accumulate on the order of 1 mg cm-2 cutin, compared to the stem of Arabidopsis, which has 0.5-10 μg cm-2. Thus, the typical 6 week period of early tomato fruit development represents a remarkably rapid and extensive phase of cuticle biosynthesis.
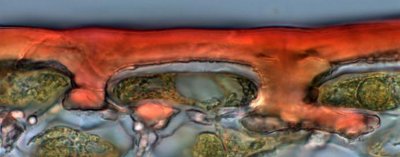
Light microscope image of the surface of an expanding tomato fruit with the cuticle stained red.
To identify new candidate proteins with a potential role in cuticle biosynthesis, and particularly those that are secreted to the apoplast, we are generating a more comprehensive inventory of proteins that are associated with the outermost surface tissues of plant organs, we are using a range of complementary protein fractionation strategies (1D gel-based and liquid chromatography) coupled with several sensitive mass spectrometry (MS) platforms.
We are also characterizing a number of tomato fruit cuticle mutants and have developing a new 3D imaging technique using confocal scanning laser microscopy for visualizing the architecture, deposition patterns and micro-structure of plant cuticles. This has allowed 3-D cuticle modeling based on the reconstruction of serial optical sections and its use in the identification of several previously unreported features of the tomato fruit cuticle. This information, together with the proteome study is providing new insights into the molecular processes underlying cuticle formation and restructuring.

A 3-D rendering of the tomato fruit (cv. M8) cuticle.
| Objectives |
- Isolate and identify proteins from the surface of expanding tomato fruits at the time of maximal cuticle biosynthesis, using solvent dips followed by gel- and liquid chromatography-based separation and several MS approaches
- Identify potentially secreted proteins and characterize their expression patterns during fruit ontogeny
- Incorporate this information into related studies of the tomato fruit cuticle t develop a more detailed understanding of cuticle deposition and restructuring
| Data Sets |
| Publications |

- Yeats, T.H. and Rose, J.K.C. (2008) The biochemistry and biology of extracellular plant lipid-transfer proteins (LTPs). Protein Science 17: 191-198.
- Buda, G.J., Isaacson, T., Matas, A.J., Paolillo, D.J. and Rose, J.K.C. (2009) Three dimensional imaging of plant cuticle architecture using confocal scanning laser microscopy. The Plant Journal 60: 378-385.
- Isaacson, T., Kosma, D.K., Matas, A.J., Buda, G.J., He, Y., Yu, B., Pravitasari, A., Batteas, J.D., Stark, R.E., Jenks, M.A. and Rose, J.K.C. (2009) Cutin deficiency in the tomato fruit cuticle consistently affects resistance to microbial infection and biomechanical properties, but not transpirational water loss. The Plant Journal 60: 363-377.
- DeBono, A., Yeats, T., Rose, J.K.C., Bird, D., Jetter, R., Kunst, L. and Samuels, L. (2009) LTPG is a GPI-anchored lipid transfer protein required for export of lipids to the plant surface. The Plant Cell 21:1230-1238.
- Matas, A.J., AgustÃ, J., Tadeo, F.R., Talon, M. and Rose, J.K.C. (2010) Tissue specific transcriptome profiling of the citrus fruit epidermis and subepidermis using laser capture microdissection. Journal of Experimental Botany 61: 3321-3330.
- Yeats, T.H., Howe, K.J., Matas, A.J., Buda, G.J., Thannhauser, T.W. and Rose, J.K.C. (2010) Mining the tomato fruit cuticular and epidermal cell wall proteome for proteins associated with cuticle biogenesis. Journal of Experimental Botany 61: 3759-3771.
- Wang, Z., Guhling, O., Yao, R., Li, F., Yeats, T.H., Rose, J.K.C., Jetter, R. (2011) Two oxidosqualene cyclases responsible for biosynthesis of tomato fruit cuticular triterpenoids. Plant Physiology 155: 540-552.
- Yeats, T.H., Buda, G.J., Wang, Z., Moyle, L.C., Jetter, R., Schaffer, A.A. and Rose, J.K.C. (2012) The fruit cuticles of wild tomato species exhibit architectural and chemical diversity, providing a new model for studying the evolution of cuticle function. The Plant Journal 69: 655-666.
- Yeats, T.H., Martin, L.B.B., Viart, H. M-F., Isaacson, T., He, Y., Zhao, L., Matas, A.J., Buda, G., Domozych, D.S. and Rose, J.K.C. (2012) The identification of cutin synthase: formation of the plant polyester cutin. (In press Nature Chemical Biology).